Helper T cell
※ Download: Cytotoxic t cell activation
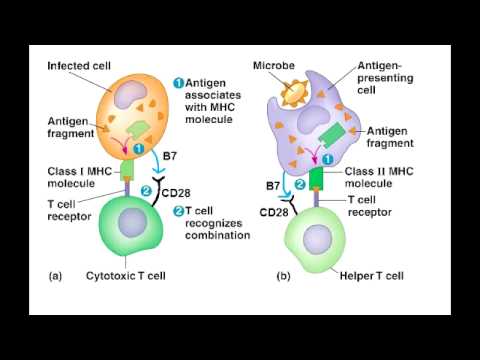
CD8 cells also require the same co-stimulatory signal as CD4 cells B7 binding to CD28. Cytotoxic T cells have been implicated in the progression of : depletion of knee joint macromolecules such as by cytotoxic T cells and has been observed in a rat model of the disease. Helper T cells themselves, however, can only function when activated to become effector cells. CD8+ T cells are recognized as T C cells once they become activated and are generally classified as having a pre-defined cytotoxic role within the immune system.

Thus, the decision of naïve helper T cells to differentiate into T H1 or T H2 effector cells influences the type of that will be mounted against the —whether it will be dominated by activation or by antibody production. We see later that CD40 ligand is also used by helper T cells to activate B cells. Positive selection means selecting those TCRs capable of recognizing self MHC molecules. These foreign proteins are selected by the antigen receptors on the surface of the B cell and are ingested by.
Cytotoxic T cells - The immune system is a complex arrangement of cells and molecules that preserve the integrity of the organism by elimination of all elements judged dangerous. Within the immune system, a humoral and a cellular as well as an innate and an adaptive arm can be differentiated.
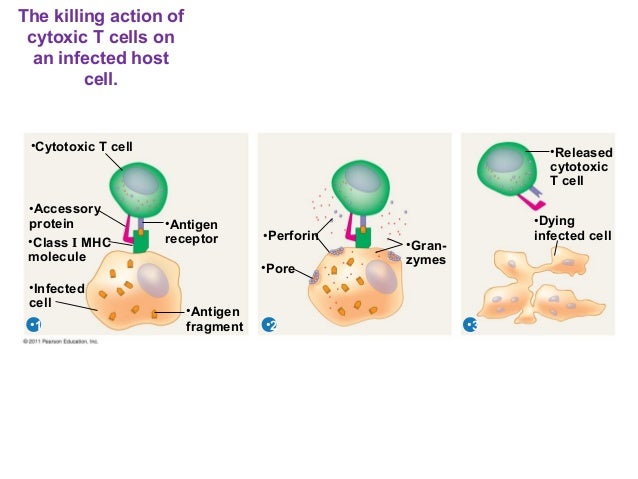
A cytotoxic T cell also known as T C, cytotoxic T lymphocyte, CTL, T-killer cell, cytolytic T cell, CD8+ T-cell or killer T cell is a a type of that kills cells, cells that are infected particularly with , or cells that are damaged in other ways. Most cytotoxic T cells express TCRs that can recognize a specific. An antigen is a molecule capable of stimulating an immune response, and is often produced by cancer cells or viruses. Antigens inside a cell are bound to molecules, and brought to the surface of the cell by the class I MHC molecule, where they can be recognized by the T cell. If the TCR is specific for that antigen, it binds to the complex of the class I MHC molecule and the antigen, and the T cell destroys the cell. In order for the TCR to bind to the class I MHC molecule, the former must be accompanied by a called , which binds to the constant portion of the class I MHC molecule. Therefore, these T cells are called CD8+ T cells. The between CD8 and the MHC molecule keeps the T C cell and the target cell bound closely together during antigen-specific activation. CD8+ T cells are recognized as T C cells once they become activated and are generally classified as having a pre-defined cytotoxic role within the immune system. However, CD8+ T cells also have the ability to make some. Development of single positive T cells in the thymus The immune system must recognize millions of potential antigens. There are fewer than 30,000 genes in the human body, so it is impossible to have one gene for every antigen. Instead, the DNA in millions of white blood cells in the bone marrow is shuffled to create cells with unique receptors, each of which can bind to a different antigen. Some receptors bind to tissues in the human body itself, so to prevent the body from attacking itself, those self-reactive white blood cells are destroyed during further development in the , in which is necessary for its development and activity. TCRs have two parts, usually an alpha and a beta chain. Some TCRs have a gamma and a delta chain. If that rearrangement is successful, the cells then rearrange their alpha-chain TCR DNA to create a functional alpha-beta TCR complex. This highly-variable genetic rearrangement product in the TCR genes helps create millions of different T cells with different TCRs, helping the body's immune system respond to virtually any of an invader. The vast majority of express alpha-beta TCRs αβ T cells , but some T cells in epithelial tissues like the gut express gamma-delta TCRs , which recognize non-protein antigens. They will differentiate into either CD4+ or CD8+ depending on which MHC is associated with the antigen presented MHC1 for CD8, MHC2 for CD4. In this case, the cells would have been presented antigen in the context of MHC1. Positive selection means selecting those TCRs capable of recognizing self MHC molecules. Only those T cells that bind to the MHC-self-antigen complexes weakly are positively selected. Those cells that survive positive and negative selection differentiate into single-positive T cells either CD4+ or CD8+ , depending on whether their TCR recognizes an MHC class I-presented antigen CD8 or an -presented antigen CD4. It is the CD8+ T-cells that will mature and go on to become cytotoxic T cells following their activation with a class I-restricted antigen. In this immunofluorescence image, a group of killer T cells outer three is engaging a cancer cell centered one. A patch of signaling molecules pink that gathers at the site of cell-cell contact indicates that the CTL has identified a target. Lytic granules red that contain cytotoxic components then travel along the microtubule cytoskeleton green to the contact site and are secreted, thus killing the target. With the exception of some cell types, such as non- cells including , Class I MHC is expressed by all cells. When these cells are infected with a or another , the cells degrade foreign proteins via. These result in peptide fragments, some of which are presented by MHC Class I to the TCR on CD8+ T cells. The activation of cytotoxic T cells is dependent on several simultaneous interactions between molecules expressed on the surface of the T cell and molecules on the surface of the APC. For instance, consider the two signal model for T C cell activation. Signal T cell APC Description First Signal peptide-bound molecule There is a second interaction between the coreceptor and the class I MHC molecule to stabilize this signal. Second Signal molecule on the T cell either or also called B7-1 and B7-2 CD80 and CD86 are known as for T cell activation. This second signal can be assisted or replaced by stimulating the T C cell with cytokines released from. A simple activation of naive CD8 + T cells requires the interaction with professional antigen-presenting cells, mainly with matured. To generate longlasting and to allow repetitive stimulation of cytotoxic T cells, dendritic cells have to interact with both, activated CD4 + and CD8 + T cells. While in most cases activation is dependent on TCR recognition of antigen, alternative pathways for activation have been described. For example, cytotoxic T cells have been shown to become activated when targeted by other CD8 T cells leading to tolerization of the latter. Once activated, the T C cell undergoes clonal expansion with the help of the cytokine IL-2 , which is a growth and factor for T cells. This increases the number of cells specific for the target antigen that can then travel throughout the body in search of antigen-positive. Through the action of perforin, granzymes enter the cytoplasm of the target cell and their function triggers the cascade, which is a series of cysteine proteases that eventually lead to programmed cell death. A second way to induce apoptosis is via cell-surface interaction between the T C and the infected cell. When a T C is activated it starts to express the surface protein FasL Apo1L CD95L , which can bind to Apo1 CD95 molecules expressed on the target cell. However, this Fas-Fas ligand interaction is thought to be more important to the disposal of unwanted during their development or to the lytic activity of certain T H cells than it is to the cytolytic activity of T C effector cells. Engagement of Fas with FasL allows for recruitment of the death-induced signaling complex DISC. The Fas-associated death domain FADD translocates with the DISC, allowing recruitment of procaspases 8 and 10. These caspases then activate the effector caspases 3, 6, and 7, leading to cleavage of death substrates such as , lamin B1, lamin B2, PARP , and DNA-activated protein kinase. The final result is apoptosis of the cell that expressed Fas. See also: During hepatitis B virus HBV infection cytotoxic T cells play an important pathogenic role. They contribute to nearly all of the liver injury associated with HBV infection and, by killing infected cells and by producing antiviral cytokines capable of purging HBV from viable hepatocytes, cytotoxic T cells also eliminate the virus. Cytotoxic T cells have been implicated in the progression of : depletion of knee joint macromolecules such as by cytotoxic T cells and has been observed in a rat model of the disease. Asian Journal of Microbiology, Biotechnology and Environmental Sciences.
As levels fall and the response subsides, effector cells are deprived of the antigen and stimulation that they need to survive, and the majority die by. When these cells are infected with a or anotherthe cells degrade foreign proteins via. For example, cytotoxic T cells have been shown to become activated when targeted by other CD8 T cells leading to tolerization of the latter. A simple activation of naive CD8 + T cells requires the interaction with professional antigen-presenting cells, mainly with matured. A second way to induce apoptosis is via cell-surface interaction between the T C and the infected cell. So whatever antigen activated the B-Cell through an interaction with the BCR will also be recognized by the antibody. If the T cell receives only signal 1, it is usually deleted or inactivated.



